

Quantum mechanics dictates that the energy levels of atomic hydrogen are discrete-just from the way the math works out. Why does the hydrogen spectrum have so few, widely spaced peaks? I apologize if I misunderstand, but it seems like you are asking two questions. There is also above threshold ionization, but without doing any calculations I am not sure how intense these effects are in terms of directly contributing to UV/vis spectra if the $x$-axis is electron energy in eV and the $y$-axis is related to excitation probability, the effect is most likely negligible in practice.
#Atomic emission spectrum vs continuous spectrum free
Any time there are free electrons, energy levels may be continuous. Note that this is for a single atom for a collection of atoms approaching a bulk phase, you may have continuous spectra under other situations.

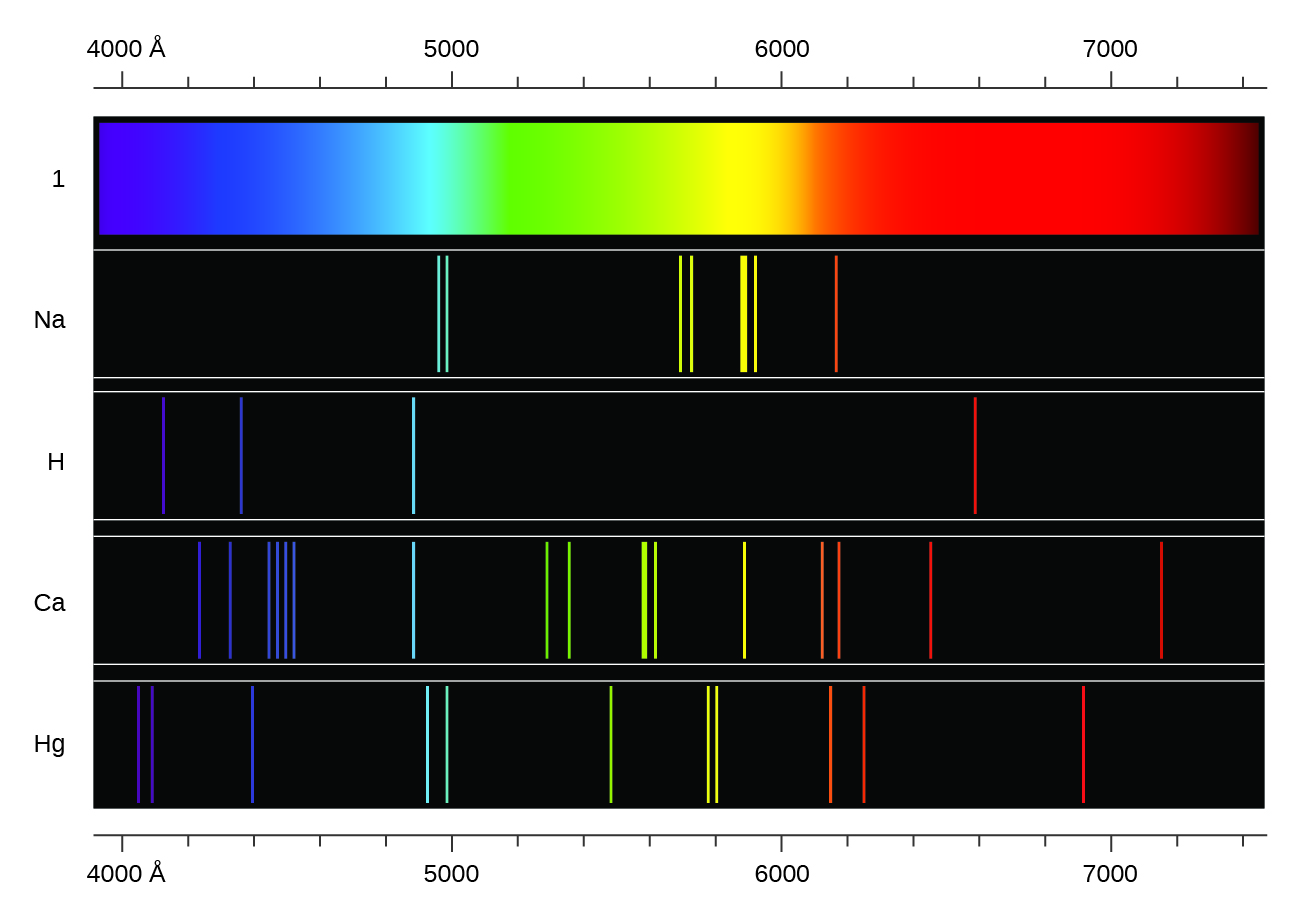
Any potential continuous band structure at higher energies due almost entirely to the free electron in the continuum, or you could think of any point in that spectral region being composed of two contributions, one being the fixed electron-proton interaction energy and the other being related to the kinetic energy of the free electron. So there is only one peak in the hydrogen emission and exitation spectra directly resulting from the interaction between the electron and the proton ( related). Applying more energy will also strip the electron from the proton, but the excitation from $n = 1$ to $n = \infty$ will still occur at the same energy, and likewise for the absorption from $n = \infty$ to $n = 1$. Past that point, what do the spectra look like? Certainly there will be peaks at the ionization energy, which is at a wavelength of about 91 nm. This is visual confirmation that as energy level spacing decreases, lines in spectra get closer together. Since energy is inversely proportional to wavelength, the higher-energy excitations are toward the left of the spectra. The infinity level represents the point at which ionization of the atom occurs to form a positively charged ion.Īt this point it is important to distinguish between hydrogen's absorption/excitation spectrum and its emission/deexcitation spectrum (taken from here): If the electron exceeds that energy, it is no longer a part of the atom. The infinity level represents the highest possible energy an electron can have as a part of a hydrogen atom. See this answer for how this arises mathematically.įor more details on the hydrogen atom, see here: In the case of the hydrogen atom, that is the Rydberg constant. However, there comes a point where the electron that's being excited is no longer bound to the atom that would be the first ionization potential (IP) or ionization energy. If you look closely enough, they are still discrete. The implication is that to a certain resolution, line spectra appear to become continuous once the energy level spacing and the excitation energies become close together. Xenon, in contrast, has many closely-spaced peaks because it has 54 electrons (so it has many more accessible energy levels than hydrogen). The reason why hydrogen's peaks are so spaced out is that it has so few degrees of freedom (just the one electron).
I'm studying emission spectrums at school right now, and my teacher said we're going to do a "flame test" for our astronomy course next week, but I have no idea what that is.Quantum mechanics dictates that the energy levels of atomic hydrogen are discrete-just from the way the math works out. Your instructor must have mentioned to you about Bunson and Kirchhoff who first studied the atomic emission spectrum by using a burner flame and a prism. How it works is that when an atom or molecule is heated, it emits radiation which can be seen as a spectrum of bright lines. Because if an x-ray is an emission spectrum, then why aren't we able to see it? bear78 February 4, A flame test is a test where an element is literally held to a flame so that it emits a spectrum of color and by that color, you can determine what that element is. I had heard that an x-ray is also an emission spectrum, but after reading this I'm kind of doubting it. We can see sunlight because it has the right wavelength, but we can't see radio waves.
an X-ray's wavelength is too short for us to see. We're not able to see x-rays because not all spectrum is visible. So you can think of x-rays as a single piece that makes up an emission spectrum. When electromagnetic radiation comes together in an arrangement, its called an electromagnetic spectrum. Serenesurface February 8, Actually an x-ray is not an emission spectrum, its electromagnetic radiation.


 0 kommentar(er)
0 kommentar(er)
