

We also present original data from a 32-country survey (n=26 758) on potential acceptance of COVID-19 vaccines, conducted from October to December, 2020. Although specific datapoints are subject to change as the pandemic response progresses, the dashboard will continue to provide a useful lens through which to analyse the key issues affecting the use of COVID-19 vaccines. We use a traffic-light system to signal the potential contributions of each candidate to achieving global vaccine immunity, highlighting important trade-offs that policy makers need to consider when developing and implementing vaccination programmes. To guide our review, we developed a dashboard to highlight key characteristics of 26 leading vaccine candidates, including efficacy levels, dosing regimens, storage requirements, prices, production capacities in 2021, and stocks reserved for low-income and middle-income countries. In this Health Policy paper, we review potential challenges to success in each of these dimensions and discuss policy implications. Yet having licensed vaccines is not enough to achieve global control of COVID-19: they also need to be produced at scale, priced affordably, allocated globally so that they are available where needed, and widely deployed in local communities. Regulators in numerous countries have authorised or approved COVID-19 vaccines for human use, with more expected to be licensed in 2021.
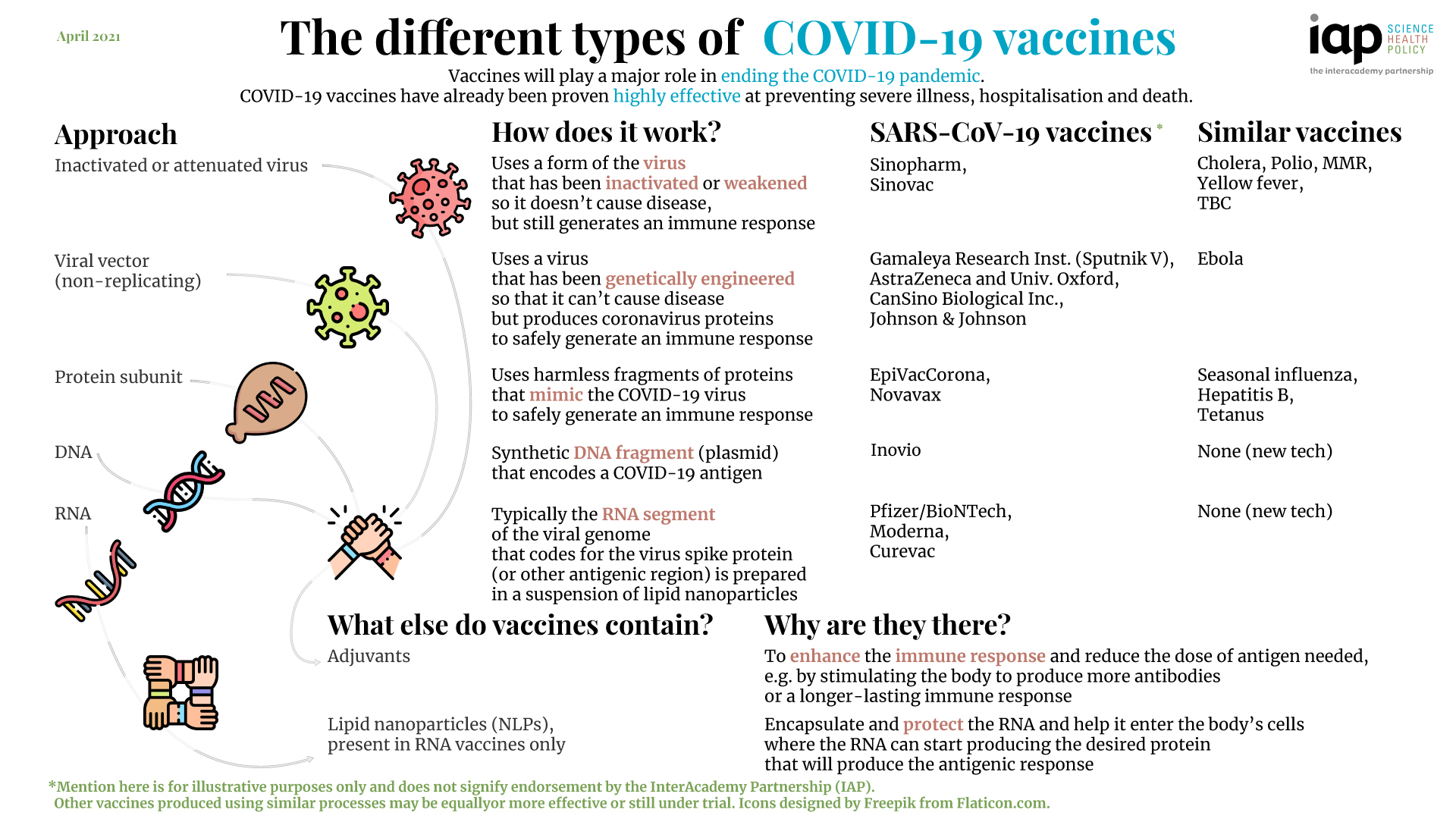
The COVID-19 pandemic is unlikely to end until there is global roll-out of vaccines that protect against severe disease and preferably drive herd immunity.


 0 kommentar(er)
0 kommentar(er)
